Revolutionizing Lab Lyophilization: Explore Martin Christ Freeze Dryers
Lyophilisation or freeze-drying is the most product-friendly drying method of all. The underlying physical phenomenon of sublimation means a direct transition from the solid to the vapour state, bypassing the liquid aggregate state. The frozen product is thus dried under vacuum without thawing.
Martin Christ Freeze Dryers is a world leader in the development and manufacturing of freeze-dryers (lyophilizers) with over 75 years of experience.
Our product portfolio encompasses laboratory lyophilization units, pilot systems and production systems, as well as vacuum concentrators for an extremely wide range of applications and process requirements.
Our product categories in Freeze drying
What is Freeze Drying / Lyophilization ?
Freeze Drying (=Sublimation) means drying frozen material (mostly water-based substances) under vacuum without going through the liquid state. The underlying physical phenomenon of sublimation means a direct transition from the solid to the vapour state, bypassing the liquid aggregate state. The frozen product is thus dried under vacuum without thawing.
Vacuum concentrators use the combination of heat, vacuum and centrifugal force to evaporate liquid samples. The process is used for evaporation, drying, purification and particularly fast concentration. The principle exploits the fact that the sample boils at room temperature and a pressure of a few hPa without being frozen. Nevertheless, the sample is not thermally stressed under vacuum.
What are the advantages of freeze drying over other sample preparation or drying methods?
Freeze-drying is the ‘gentlest’ and then t
he most product-friendly drying method of all
method to conserve substances and materials.
Lyophilisation is the smoothest available drying technique; drugs are often thermosensitive and might react with water over longer periods (hydrolysis).
There is no degradation or modification of the molecules, and therefore no loss of biological activity.
The product can easily be stored, resulting in reconstitution in seconds.
Mastering Freeze Drying and Vacuum Concentration (RVC): Basics & Fundamentals
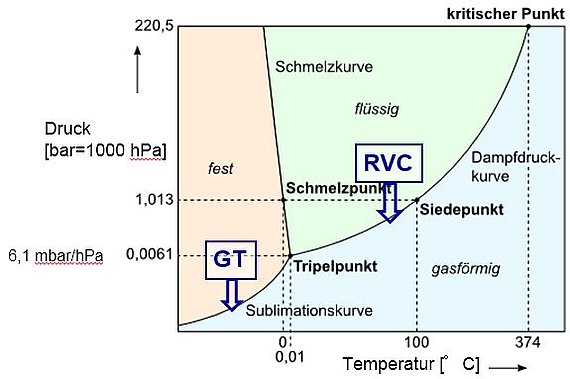
Vacuum concentration and freeze drying are related methods used for the gentle drying or preservation of thermally sensitive materials. This often involves the removal of aqueous media. As can be seen from the state diagram of water, which can also serve as an example for other solvents, above a certain process pressure (in this case 6.1 hPa for H2O) drying occurs from the liquid phase. At pressures below this level, the water temperature drops below 0 °C.
Drying takes place directly - bypassing the liquid phase - from the ice state. The triple point (here H2O: p=6.1 hPa, T=0 °C) represents, to a certain extent, the boundary state between vacuum concentration (drying) and freeze-drying. One observes the simultaneous occurrence of ice, water and steam in the recipient. The following table contains essential characteristics of both processes.
Comparison: Vacuum Concentration vs. Freeze Drying Parameters
| Criterion
|
Vacuum concentration
|
Freeze drying
|
|
Evaporation temperature range (gentle process?)
|
–5 °C to + 20 °C
|
–60 °C to 0 °C
|
|
Substances
|
Usually dissolved; residue is a powder or a crystalline substance
|
Solids (including ceramics, meat, archaeological objects, bones, plants and books)
|
|
Solvents
|
Various solvent types (some exotic)
|
Aqueous solutions, only small quantities or special solvents
|
|
Water/solvent content
|
Approx. 5% final content
|
Low residual moisture possible (< 1%)
|
|
Process time
|
Minutes to hours 0.5 – 3 days
| 0.5 – 3 days ; several weeks in some cases |



