Révolutionner la FIV : les incubateurs MIRI® Multiroom d'Esco Medical établissent la norme en matière de précision, de flexibilité et de conception sans perturbation
Esco Medical, leader dans la fabrication et l'innovation d'équipements de haute qualité pour l'industrie de la FIV, propose l'un des concepts d'incubateur les plus uniques.
Entrant sur un marché concurrentiel depuis 2014 avec certains fabricants d'équipements de FIV bien connus, Esco Medical a présenté un système multichambre intensivement conçu pour répondre aux besoins des embryologistes.
Plus que de simples chambres individualisées, la ligne d'incubateurs MIRI® se targue d'une excellente uniformité de température et de gaz, ainsi que de temps de récupération.
Tous les modèles d'incubateurs MIRI® disposent également d'une chambre de mélange de gaz qui utilise du gaz pur (100 % CO2, 100 % N2), ce qui permet une flexibilité et un contrôle précis de la composition de la phase gazeuse dans les chambres.
L'air de recirculation est maintenu propre et exempt de composés nocifs grâce à son filtre HEPA/VOC et à la lumière UVC (254 nm) qui stérilise le flux d'air.
Outre ces caractéristiques essentielles, la conception de MIRI® Multiroom garantit également l'absence de perturbations dans les chambres voisines lorsque l'une d'entre elles s'ouvre, ce qui en fait l'un des meilleurs incubateurs du secteur à l'heure actuelle.
Nos produits

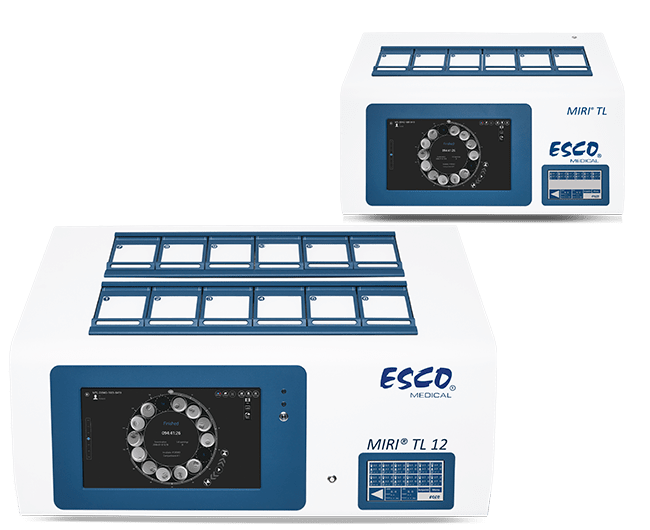
The MIRI® II Multiroom Incubator dedicates one chamber for one patient. IVF lab can choose from models having 6 or 12 chambers that are completely independent of each other. Any disruption on one chamber (e.g. temperature drop after opening the lid) has zero impact on the rest of the system. It has a touchscreen PC to control and monitor important parameters (temperature, gas concentration) simultaneously and give visual and audible alarm to signal critical conditions.
FEATURES:
1. One Chamber - One Patient
2. Superior gas system
3. Heated lid
4. Fast temperature and gas recovery
5. Touchscreen PC
6. Optimized heating plates
7. Easy external validation
8. Full-featured data logging software
SAFE Sens pH monitoring system for IVF culture media is integrated as a standard feature. In the US, every MIRI® II-12 unit will come equipped to easily turn on the pH monitoring feature. The SAFE Sens provides the following benefits over conventional pH tools:
(a) a decrease in time spent on pH protocols,
(b) data trending to optimize media pH equilibration time,
(c) having out-of-range email alert system, and
(d) being non-invasive due to the non-usage of petri dish and the constant opening of the incubator.

Multiroom incubator for embryo culture with 6 independent chambers, preventing cross contamination without causing any disturbance to the neighboring chambers even when the lids are opened/closed.
The MIRI is the actual standard system in new IVF labs.
DELIVERY INCLUDES:
-1 pc HEPA+VOC filter
-2 pcs External in-line filters
-2 pcs Fast connector with 15cm silicone tubing
-1 pc Data extension cable (1.5m)
-1 pc USB key with Data logging software
-1 pc power cable
-6 pcs heating optimization plates for Falcon, Nunc, or Vitrolife (please specify at the order the type of plate holder to be included)
FEATURES:
Six (6) completely independent chambers
Six independent MIRI® chambers, with precise temperature and gas regulation: Opening any chamber does not affect the environment in other chambers. Temperature or gas fluctuation caused by opening the lid have no impact on the rest of the system.
Superior gas system
Works with premix gas or 100% CO2 gas, with NDIR CO2 sensor to regulate CO2 levels. HEPA-VOC gas filtration system ensure clean gas recirculation
Heated lid
Heated chamber lids prevent condensation, enhance temperature regulation/recovery, and ensure temperature uniformity between the top and bottom of the chamber.
Fast temperature and gas recovery
Temperature recovery <1 min
Gas recovery <3 mins.
LED display
Large easy to read LED display and easy to navigate menu.
Optimized heating plates
Heating plate inserts to fit commonly used embryo culture dishes.
Easy external validation
Gas sampling ports for each chamber and independent PT-1000 sensors allow external quality evaluation of gas levels and temperature in each chamber.
MIRI® Data Logger
Continually monitors and documents gas flow, gas concentration and pressure, and temperature regulation.
Audible alarms for out of range parameters, with alarm logs documented in the Data Logger.
Space-saving the touchscreen tablet and stacking frame
Maximise the laboratory workplace by letting go of bulky computers and saving space with a tablet and a MIRI® stacking frame. Real time parameters such as temperature, gas concentration points, gas input pressures, gas flow rates, and even pH measurement can be conveniently viewed through the touchscreen tablet.
Continuous pH monitoring
Optional SAFE Sens integration for continuous pH monitoring without opening the chamber.

High-efficiency particulate air (HEPA) filters remove particles smaller than 0.3 µm. Filters to remove volatile organic compounds (VOC – large group of carbon-based chemicals that easily evaporate at room temperature), which could potentially jeopardize embryo culture procedures. It has been known that the air quality in IVF laboratory can have higher levels of VOCs than outdoor environments air. The impact of VOC on embryo culture will significantly affect on embryo cleavage rate, embryo development and blastocyst formation rate.
ESCO HEPA filter with efficiency of 99.99% at 0.3 microns.
ESCO VOC filter to remove any compounds that are harmful to embryo development.
To be replaced every 3 months
Accessory for:
-MRI-6A10
-MRI-TL