Maak je klaar om de grenzen van EV-detectie te doorbreken! Ga Dieper!
Extracellulaire vesikels zijn belangrijke spelers in intercellulaire communicatie en spelen een significante rol in de ontwikkeling van immuunresponsen, weefselregeneratie, tumorgroei en meer. Hun analyse wordt echter bemoeilijkt door de beperkingen van traditionele cytometrie bij het detecteren van EV's kleiner dan 100 nm.
Met de CytoFLEX nano verlaag je de detectielimieten, zodat je meer kunt bereiken.
Een nieuwe benadering van nanoschaal flowcytometrie met de BECKMAN COULTER CytoFLEX nano
Nanoscale flow cytometry is een geavanceerde techniek die de principes van flowcytometrie combineert met nanotechnologie. Het stelt de analyse van deeltjes op nanoschaal in staat, waardoor waardevolle informatie over hun grootte, samenstelling en oppervlakte-eigenschappen wordt verkregen. Nanoscale flow cytometry heeft talrijke toepassingen in verschillende vakgebieden, waaronder biologie, geneeskunde en materiaalkunde. Het stelt onderzoekers in staat om nanopartikels, extracellulaire vesikels (EV's) en andere kleine deeltjes met grote precisie en gevoeligheid te analyseren en te karakteriseren. Bovendien biedt nanoscale flow cytometry ook perspectieven voor de ontwikkeling van diagnostische hulpmiddelen en gerichte medicijnafgiftesystemen. De mogelijkheid om nanopartikels op basis van hun kenmerken te analyseren opent nieuwe mogelijkheden voor gepersonaliseerde geneeskunde en nanogeneeskunde.
Vraag het onze experts

De CytoFLEX nano is de eerste speciaal gebouwde nanoschaal flowcytometer die nanopartikels kan detecteren, zoals extracellulaire vesikels (EV's) die minstens zo klein zijn als 40 nm, terwijl tegelijkertijd multiparametrische fluorescentiedetectie wordt uitgevoerd. De software-interface van de CytoFLEX nano, CytExpert nano, biedt de verfijning om het onbekende in het nanoschaalbereik te verkennen, terwijl het de gebruiksvriendelijkheid van het CytoFLEX-platform biedt. Op deze manier wordt het verkrijgen van antwoorden op uitdagende onderzoeksvragen gemakkelijker dan ooit voor EV-onderzoekers.
Wat maakt CytoFLEX nano uniek?
Bouwend op dezelfde principes als het CytoFLEX-platform, combineert de CytoFLEX nano de mogelijkheden van verschillende methoden in één enkel platform, wat de detectie van nanopartikels zo klein als 40 nm mogelijk maakt, de analyse van individuele EV-markers zelfs bij lage expressie (<10 antigeen kopieën) en de mogelijkheid biedt om gestandaardiseerde, snelle en nauwkeurige analyses te leveren zonder gebruik te maken van meerdere systemen.
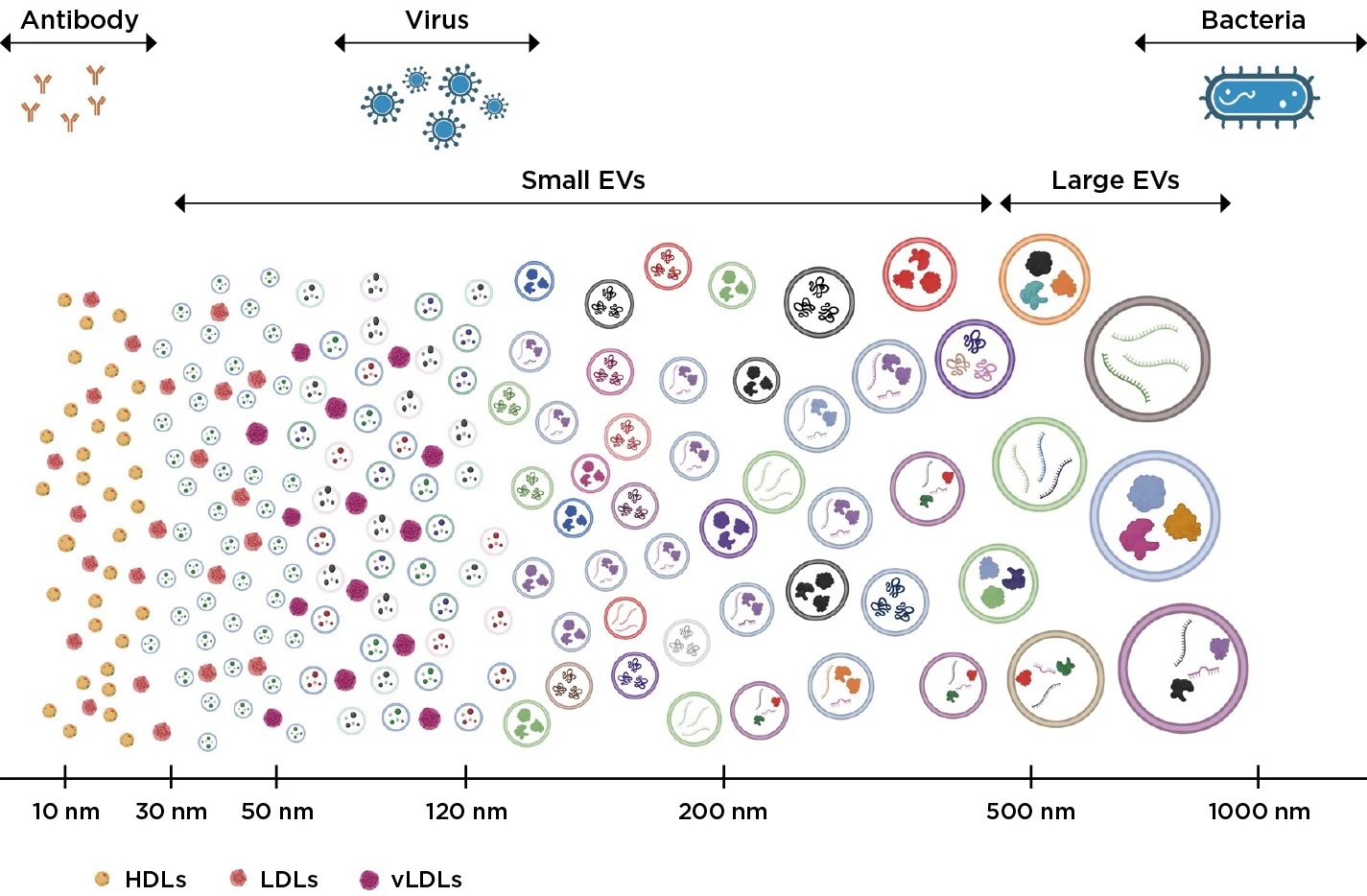
Bron Beckman Coulter - Figuur 1. De grootte van verschillende cellen en extracellulaire vesikels. De CytoFLEX Nano Flow Cytometer kan extracellulaire vesikels detecteren, karakteriseren en de grootte bepalen van vesikels zo klein als 40 nm
Belangrijkste Voordelen en Kenmerken
Gevoeligheid en Multi-Channel Detectie
6 fluorescent channels and 5 side scatter channels allow detailed analysis of even the smallest particles.
Verhoogde lasersterkte en speciaal ontworpen optische excitatiepaden verzamelen meer licht, waardoor de efficiëntie van de analyse wordt verbeterd.
Gegevenszuiverheid en minimalisatie van contaminatie
Geautomatiseerde reinigingshulpmiddelen detecteren en elimineren snel verontreinigingen, waardoor het risico op monsterverlies tot <1% wordt geminimaliseerd.
Het dagelijkse kwaliteitscontroleproces detecteert geluid van nano- en microbellen en activeert automatische spoeling en reiniging.
Geoptimaliseerde Optische en Vloeistofsystemen
Verminderde stalen- en sheathvloeistofdebieten maken het mogelijk om met microstalen te werken, wat zorgt voor een hoge nauwkeurigheid van de resultaten.
Monsterverzameling met een kleine-volume zuigerpomp zorgt voor stabiliteit en nauwkeurigheid van de telling (>90%).
Gespecialiseerde omhulvloeistof, gecombineerd met een variabele 5-nm filter, minimaliseert achtergrondruis.
Gebruiksgemak
De intuïtieve CytExpert-software maakt een gemakkelijke systeemconfiguratie en aanpassing aan de behoeften van gebruikers op elk niveau mogelijk.
Procesautomatisering vermindert de tijd en vereenvoudigt de monster voorbereiding.
De CytoFLEX nano combineert alles in slechts één instrument, waardoor telling, karakterisering en groottebepaling mogelijk zijn
TOTAAL TESTBEHEER
De bepaling van de deeltjesgrootte met behulp van flowcytometrie vereist kalibratie met een passend gekarakteriseerde referentiedeeltje in combinatie met een kalibratiemethode. Latex polystyreen deeltjes zijn het favoriete hulpmiddel voor deze taak vanwege hun NIST-traceerbare aard en de betrouwbaar consistente productie. Via de kalibratiemethode die aan elke CytoFLEX nano flowcytometerklant wordt aangeleerd, zullen de gegevens betrouwbaar rapporteerbaar zijn in absolute eenheden voor methodevergelijking en correlatie.
TEL
De meest robuuste telmethoden zijn gebaseerd op sterk gecontroleerde volumetrische afgifte van monsters in plaats van handmatig laden van monsters met de impliciete variabiliteit. De CytoFLEX nano flow cytometer stelt gebruikers in staat om hun monsterverwerking aan te passen, zodat de juiste hoeveelheid monster wordt gebruikt voor de benodigde resultaten.
KENMERKEN
De mogelijkheid om nanoschaaldeeltjes te beschrijven vereist een ongeëvenaarde gevoeligheid. Om dit mogelijk te maken, heeft de CytoFLEX nano flowcytometer het optische en vloeistofsysteem volledig herontworpen om de detectie van zelfs de kleinste hoeveelheden fluorescentie over 6 detectiekanalen te maximaliseren, opgewonden door 4 collineaire lasers.
Verken het volledige plaatje van uw EV-experiment met de CytoFLEX nano Flow Cytometer.
Deze speciaal gebouwde flowcytometer biedt een grotere gevoeligheid, consistente instrumentprestaties en flexibiliteit om uw monster te bestuderen.
Gevoeligheid
Met een ongeëvenaarde gevoeligheid voor grootte van 1µm tot 40nm* en een resolutie van 10nm,** stelt de CytoFLEX nano flowcytometer in staat om kleinere EV-populaties en hun laag-abundance lading te detecteren.
Flexibiliteit
Open uw onderzoek met 6 fluorescerende kanalen en 5 zijverstrooiingskanalen. Verzamel alle noodzakelijke gegevens met minder beperkingen.
Maximale Consistentie
Het handhaven van een contaminatievrije, robuuste instrumentprestaties is cruciaal bij het werken met kleine deeltjes. Verkrijg reproduceerbare resultaten waarop je kunt vertrouwen met >90% volumetrische telprecisie en <1% carryover tussen monsters dankzij geautomatiseerde kwaliteitscontroles.
Bekendheid
Bouwend op dezelfde principes als het CytoFLEX-platform, beschikt de CytoFLEX nano flowcytometer over een vergelijkbare afdruk en gebruiksvriendelijke software-interface.
Geïnteresseerd in het leren hoe je jouw onderzoek naar Extracellulaire Vesikels kunt bevorderen?
Ontdek hoe de BECKMAN COULTER CytoFLEX nano Flow Cytometer de eerdere grenzen van detectiecapaciteiten doorbreekt, waardoor onderzoekers worden uitgerust met gegevens die ze voorheen nooit hadden kunnen verkrijgen.
Bij het kiezen van een flowcytometrie-instrument zijn er drie belangrijke factoren om te overwegen:
Het tellen, bepalen van de grootte en karakteriseren van extracellulaire vesikels (EV's) biedt onderzoekers diepere inzichten in de gezondheid van cellen en organen en ziekteprofielen. EV's kunnen echter moeilijk te analyseren zijn, vanwege hun kleine formaat en heterogeniteit.
De nood aan standaardisatie
Tot nu toe hebben wetenschappers meerdere technieken moeten gebruiken om EV's nauwkeurig te tellen, te karakteriseren en de grootte ervan te bepalen, om de inzichten te krijgen die ze nodig hebben. Als gevolg hiervan zijn workflows die tijdrovend zijn en een slechte herhaalbaarheid hebben vaak vereist.
Webinar: Eerste ervaringen met de CytoFLEX nano
Dit webinar bevat Alfonso Blanco, Directeur van Flow aan University College Dublin, die voorlopige resultaten zal presenteren die onderzoekers die extracellulaire vesikels (EV's) bestuderen, hebben verkregen met behulp van de CytoFLEX nano Flow Cytometer. Dit webinar verkent de grenzen van de flowcytometrie-technologie en schetst hoe de CytoFLEX nano flow cytometer is ontworpen om deze beperkingen aan te pakken in een robuust en consistent systeem dat in staat is om resultaten tussen laboratoria te standaardiseren. Onze expert spreker, Alfonso Blanco, bespreekt de voordelen van het gebruik van fluorescentie in combinatie met meerdere verstrooiingen voor verdere karakterisering van nanopartikels en extracellulaire vesikels. Bekijk de herhaling
Persoverzicht: Wat Ze Zeggen Over Recente Vooruitgangen en Inzichten in Onderzoek naar Extracellulaire Vesikels
In het artikel, "Ontwikkelingen in het Onderzoek naar Extracellulaire Vesikels (EV) Bieden een Veelbelovende Toekomst", gepubliceerd in Technology Network - juli 2024, spreekt Matthew Goff, Productmanager bij Beckman Coulter, over de volgende onderwerpen:
- Traditional challenges in EV research
- Emergence of new technologies
- The importance of workflow automation
- How nanoscale technology is accelerating EV research
"....Met meer duidelijkheid in de resultaten bieden EV's geavanceerde diagnostische, prognostische, theranostische en therapeutische procedures, terwijl ze tegelijkertijd de invasieve aard van monsterverzameling verminderen. Het monitoren van ziekteprogressie of het creëren van op maat gemaakte therapeutische modellen is ongelooflijk krachtig, maar het vereist specificiteit in analytische methoden. Dit is eindelijk beschikbaar voor een verscheidenheid aan onderzoekers. “Updating this workflow is really an exciting moment in research....”
Beckman Coulter Life Sciences revolutioneert de analyse van nanopartikels met de lancering van de CytoFLEX Nano Flow Cytometer.
Artikel gepubliceerd in Technology Network: 28 maart 2024
“...De CytoFLEX nano Flow Cytometer doorbreekt de eerdere grenzen van detectiecapaciteiten en geeft onderzoekers toegang tot gegevens die ze voorheen nooit hadden kunnen verkrijgen.” Dit kan nieuwe deuren openen voor onderzoekers en doorbraakontdekkingen mogelijk maken in onze strijd tegen een groot aantal ziekten. Extracellulaire vesikels zijn overal te vinden, en nu hebben onderzoekers eindelijk een hulpmiddel om te zien wat altijd al voor hun neus was. We hebben geluisterd naar de behoeften van onze klanten om een analytisch kruispunt te creëren door de gevoeligheid te verhogen via verbeterde fluidica, optica en elektronica......”
Extracellulaire vesikels (EV's) zijn kleine membraangebonden deeltjes die door cellen in de extracellulaire omgeving worden vrijgegeven. Ze spelen belangrijke rollen in intercellulaire communicatie en zijn betrokken bij verschillende fysiologische en pathologische processen. EV's worden ingedeeld in verschillende subtypes op basis van hun biogenese en grootte, waaronder exosomen, microvesikels en apoptotische lichamen. Exosomen of kleine EV's hebben doorgaans een grootte van 30 tot 150 nm. Ze worden gevormd door de naar binnen buigende uitsteeksels van multivesiculaire lichamen (MVB's) binnen de cel, die vervolgens fuseren met het plasmamembraan, waardoor de exosomen in de extracellulaire ruimte worden vrijgegeven. Exosomen bevatten verschillende bioactieve moleculen, zoals eiwitten, lipiden, nucleïnezuren (DNA, RNA) en microRNA's, die kunnen worden overgedragen aan doelcellen, waardoor hun functie en gedrag worden beïnvloed. Microvesikels, ook wel grote EV's of micropartikels of ectosomen genoemd, zijn groter dan exosomen, met een grootte van 100 tot 1000 nm. In tegenstelling tot exosomen worden microvesikels gevormd door de naar buiten buigende uitsteeksels en het afstoten van het plasmamembraan direct. Ze dragen ook een diverse lading van eiwitten, lipiden en nucleïnezuren en kunnen deze moleculen overdragen aan ontvankelijke cellen.
Apoptotische lichamen zijn de grootste EV's, die typisch variëren van 1 tot 5 µm. Ze worden vrijgegeven door stervende cellen tijdens het proces van geprogrammeerde celdood (apoptose). Apoptotische lichamen bevatten cellulaire fragmenten, organellen en nucleair materiaal, en worden herkend en opgenomen door fagocytische cellen om hun verwijdering te vergemakkelijken.
Technologieën zoals nano flow cytometrie, elektronenmicroscopie en moleculaire profileringstechnieken zoals RNA-sequencing en proteomics worden gebruikt om EV's te bestuderen en te karakteriseren. Het begrijpen van de biologie, lading en functies van EV's biedt veelbelovende mogelijkheden om onze kennis van cellulaire communicatie en hun potentiële toepassingen in diagnostiek, therapie en regeneratieve geneeskunde te bevorderen. Momenteel is de analyse van EV's cruciaal en uitdagend. Isolatie- en zuiveringsmethoden kunnen lijden onder een lage opbrengst, contaminatie door andere deeltjes en moeilijkheden bij de standaardisatie. De heterogeniteit van EV-populaties in termen van grootte, lading en biogenese bemoeilijkt hun studie. EV's kunnen verschillende biologische activiteiten en functies vertonen, afhankelijk van hun cellulaire oorsprong en lading. Het ontcijferen van de specifieke functies en werkingsmechanismen van EV's in verschillende contexten blijft echter een uitdaging. De functionele heterogeniteit van EV's vereist een meer uitgebreide karakterisering en gestandaardiseerde functionele assays. Onderzoekers op het gebied van EV's werken actief aan het aanpakken van deze beperkingen door verbeterde isolatietechnieken te ontwikkelen, protocollen te standaardiseren en onze kennis van de biologie van EV's te bevorderen. Naarmate het veld vordert, zal het overwinnen van deze uitdagingen helpen om het volledige potentieel van EV-onderzoek en de toepassingen ervan in verschillende biomedische gebieden te ontsluiten. Een van de grootste beperkingen is de noodzaak van meerdere technieken om EV's nauwkeurig te tellen, te karakteriseren en de grootte ervan te bepalen, wat resulteert in tijdrovende en arbeidsintensievere workflows met een slechte herhaalbaarheid.
