- Analytical chemistry
- Automated Liquid Handling
- Capillary Electrophoresis
- Cell culture and Fermentation
- Cell health
- Centrifugation
- Chromatography (flash & prep)
- Cleaning
- Clinical Automation (TLA)
- Clinical IT solutions
- Clinical chemistry
- Clinical quality controls
- Color-Appearance and Physical tests
- DNA / RNA (Nucleic acids)
- Disinfection
- Electrochemistry
- Environmental monitoring
- Flow cytometry
- Flow cytometry Cell sorting
- Freeze drying
- Heating & cooling
- Hematology
- Imaging
- Immuno-Hematology
- Immunoassay
- In Vitro Fertilization (IVF)
- Lab furniture & design studies
- Liquid Handling (Manual)
- Mass spectrometers
- Material testing
- Metrology
- Microbiology (Clinical)
- Microscopy
- Moisture analysis
- Nephelometry
- POCT
- Particle characterization
- Platelet aggregation
- Protection cabinets
- Pumping
- Sample preparation
- Shakers and stirrers
- Sterilization
- Storage
- Synthesis
- Tablet testing
- Texture analyzers
- Toxicology
- Urine analysis
- Viscometry
- Water purification
- Weighing
- Capillary Electrophoresis
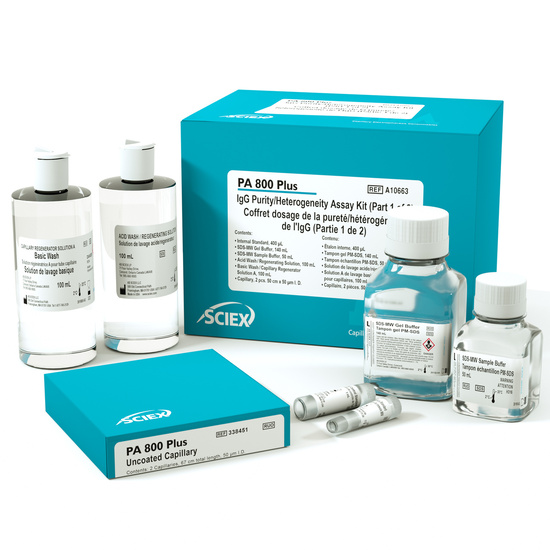
- IgG Purity/Heterogeniety Assay Kit
HOME / APPLICATIONS & TECHNIQUES /
IgG Purity/Heterogeniety Assay Kit
Reference: ABS-A10663
https://www.analis.com/shop/abs-a10663-igg-purity-heterogeniety-assay-kit-21683 https://www.analis.com/web/image/product.template/21683/image_1920?unique=689d05dIncludes IgG Control Standard 1-pack and all chemistries.
The IgG Purity/Heterogeneity Assay has been developed for researchers employed in industrial biotechnology who are developing and manufacturing IgG reagents for research, diagnostic and therapeutic use.
This assay has been specified for use on the PA 800 Plus to assess the purity and heterogeneity of IgG reagents in both a reduced and non-reduced state. The methodology involves heat-denaturing a specified concentration of IgG (both reduced and non-reduced) in the presence of SDS, and separating these proteins by size using high-resolution capillary gel electrophoresis technology. This assay will detect impurities as low as 0.1% and includes an IgG control with a designated quantity of non-glycosylated heavy chain to test both the resolution and quantitation suitability of the assay prior to running unknowns
Assay chemistry includes:
• Separation Capillary, 57 cm x 50 µm ID bare fused-silica, (2)
• SDS Gel Separation Buffer (proprietary formulation), 140 mL
• SDS Sample Buffer, 100 mM Tris-HCl, pH 9.0/1% SDS, 50 mL
• IgG Control Standard, 1 mg/mL in SDS sample buffer , 1 mL
• Internal Standard, 10 kDa protein, 5 mg/mL, 0.4 mL
• Acidic Wash Solution, 0.1 N HCl, 100 mL
• Basic Wash Solution, 0.1 N NaOH, 100 mL
• IgG Purity and Heterogeneity Analysis Guide, (1)
Related accessories, consumables & products
Automated Liquid Handling
Capillary Electrophoresis
Cell culture and Fermentation
Cell health
Centrifugation
Chromatography (flash & prep)
Cleaning
Clinical Automation (TLA)
Clinical IT solutions
Clinical chemistry
Clinical quality controls
Color-Appearance and Physical tests
DNA / RNA (Nucleic acids)
Disinfection
Electrochemistry
Environmental monitoring
Flow cytometry
Flow cytometry Cell sorting
Freeze drying
Heating & cooling
Hematology
Imaging
Immuno-Hematology
Immunoassay
In Vitro Fertilization (IVF)
Lab furniture & design studies
Liquid Handling (Manual)
Mass spectrometers
Material testing
Metrology
Microbiology (Clinical)
Microscopy
Moisture analysis
Nephelometry
POCT
Particle characterization
Platelet aggregation
Protection cabinets
Pumping
Sample preparation
Shakers and stirrers
Sterilization
Storage
Synthesis
Tablet testing
Texture analyzers
Toxicology
Urine analysis
Viscometry
Water purification
Weighing
