- Analytical chemistry
- Automated Liquid Handling
- Capillary Electrophoresis
- Cell culture and Fermentation
- Cell health
- Centrifugation
- Chromatography (flash & prep)
- Cleaning
- Clinical Automation (TLA)
- Clinical IT solutions
- Clinical chemistry
- Clinical quality controls
- Color-Appearance and Physical tests
- DNA / RNA (Nucleic acids)
- Disinfection
- Electrochemistry
- Environmental monitoring
- Flow cytometry
- Flow cytometry Cell sorting
- Freeze drying
- Heating & cooling
- Hematology
- Imaging
- Immuno-Hematology
- Immunoassay
- In Vitro Fertilization (IVF)
- Lab furniture & design studies
- Liquid Handling (Manual)
- Mass spectrometers
- Material testing
- Metrology
- Microbiology (Clinical)
- Microscopy
- Moisture analysis
- Nephelometry
- POCT
- Particle characterization
- Platelet aggregation
- Protection cabinets
- Pumping
- Sample preparation
- Shakers and stirrers
- Sterilization
- Storage
- Synthesis
- Tablet testing
- Texture analyzers
- Toxicology
- Urine analysis
- Viscometry
- Water purification
- Weighing
- POCT Hematology POCT
- HemoScreen Analyzer
HOME / APPLICATIONS & TECHNIQUES /
HemoScreen Analyzer
Reference: PIX-HS-FA-30219
https://www.analis.com/shop/pix-hs-fa-30219-hemoscreen-analyzer-129134 https://www.analis.com/web/image/product.template/129134/image_1920?unique=689d05dPixCell HemoScreen™ – Lab-Quality CBC Testing at the Point of Care
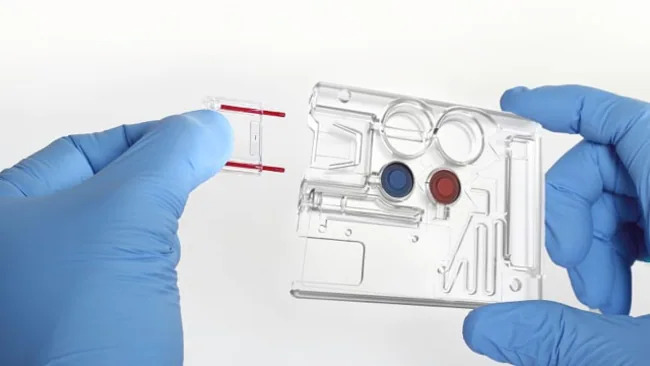
The PixCell HemoScreen™ is a compact, FDA-cleared and CE-marked hematology analyzer that delivers a 5-part differential Complete Blood Count (CBC) from a single drop of blood — in just 5 minutes. Specifically designed for true point-of-care use, it offers unmatched simplicity and accuracy, with:
- No maintenance required
- No calibration needed
- No reagent handling
- Capillary or venous blood sampling (20 µL)
- Disposable all-in-one cartridges with sealed reagents and waste
- Validated accuracy in peer-reviewed studies
The analyzer uses PixCell’s patented viscoelastic focusing technology combined with AI-powered image analysis to differentiate and count cells with high precision.
Ideal for decentralized diagnostics, the HemoScreen™ supports clinical decision-making across various settings, including:
- Emergency departments & ICUs
- Oncology infusion centers
- Psychiatry clinics (e.g., clozapine monitoring)
- Pediatrics & primary care
- Military and mobile healthcare units
With its portable format (5.5 kg) and intuitive interface, the HemoScreen™ makes hematology accessible where it matters most.
Technical information
| Parameter | Specification |
| Test parameters | 20 CBC parameters incl. 5-part diff (see below) |
| Sample type | Capillary or venous whole blood |
| Sample volume | 20 µL |
| Time to result | < 5 minutes |
| Throughput | ~10 samples/hour |
| Analyzer dimensions (H×W×D) | 30 × 17.5 × 26 cm |
| Weight | 5.5 kg (12.1 lbs) |
| Operating temperature | +17°C to +27°C |
| Humidity range | 10%–90% RH (non-condensing) |
| Power supply | 100–240 VAC, 50/60 Hz, approx. 60W |
| Operating system | Windows 10 |
| Connectivity | USB, Ethernet, HL7, POCT1-A |
| Quality control | PIX-CBC 3-level liquid control (Bio-Techne) |
| Calibration | Factory-calibrated, no user calibration needed |
| Cartridge system | Disposable, self-contained reagents and waste |
| Certifications & approvals | FDA-cleared, CE-marked, TGA-approved |
Reported Parameters:
WBC, NEU#, NEU%, LYM#, LYM%, MON#, MON%, EOS#, EOS%, BAS#, BAS%, RBC, HGB, HCT, MCV, MCH, MCHC, RDW-CV, PLT, MPV
With flagging for:
Immature granulocytes (IG), nucleated RBC, blasts, atypical lymphocytes
Automated Liquid Handling
Capillary Electrophoresis
Cell culture and Fermentation
Cell health
Centrifugation
Chromatography (flash & prep)
Cleaning
Clinical Automation (TLA)
Clinical IT solutions
Clinical chemistry
Clinical quality controls
Color-Appearance and Physical tests
DNA / RNA (Nucleic acids)
Disinfection
Electrochemistry
Environmental monitoring
Flow cytometry
Flow cytometry Cell sorting
Freeze drying
Heating & cooling
Hematology
Imaging
Immuno-Hematology
Immunoassay
In Vitro Fertilization (IVF)
Lab furniture & design studies
Liquid Handling (Manual)
Mass spectrometers
Material testing
Metrology
Microbiology (Clinical)
Microscopy
Moisture analysis
Nephelometry
POCT
Particle characterization
Platelet aggregation
Protection cabinets
Pumping
Sample preparation
Shakers and stirrers
Sterilization
Storage
Synthesis
Tablet testing
Texture analyzers
Toxicology
Urine analysis
Viscometry
Water purification
Weighing