Solutions and Early Detection Assays for Labs of All Sizes
In the Global Fight Against Neurodegenerative Diseases,We’re All in This Together, we’re with you every step of the way.
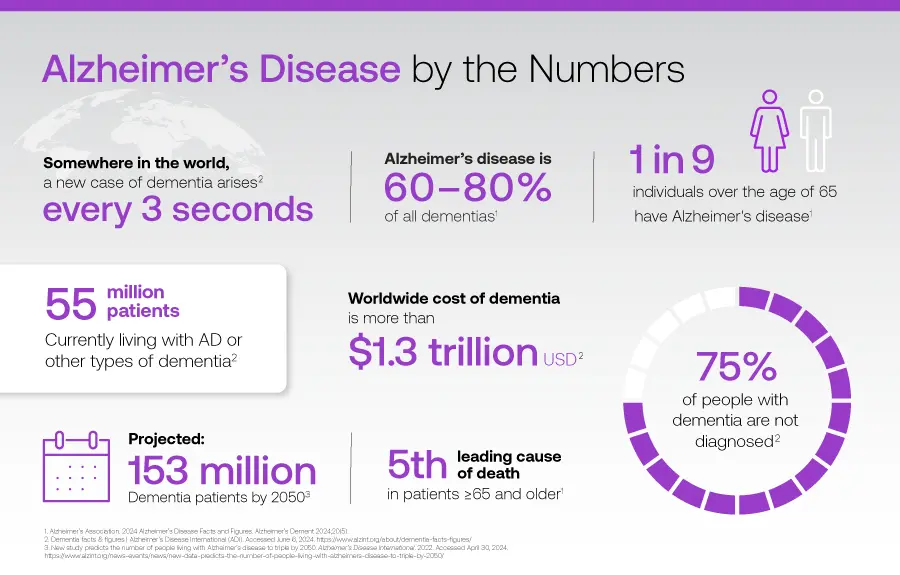
Neurodegenerative Disease by the Numbers
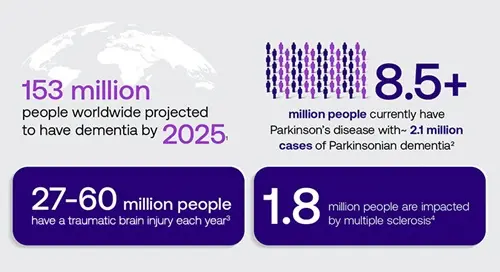
- 153 million people worldwide projected to have dementia by 20511
- 8.5+ million people currently have Parkinson’s disease with ~2.1 million cases of Parkinsonian dementia2
- 27-60 million people have a traumatic brain injury each year3
- 1.8 million people are impacted by multiple sclerosis4
NEW High-quality blood-based assays enable laboratory to early detect Alzheimer diseases in a less invasive way.
New emerging blood tests aim to detect Alzheimer in a less invasive and more accessible way. Labs Could Soon Play Leading Roles in Alzheimer’s Disease Testing. Rather than relying on invasive and often hard-to-access methods like CSF testing and PET scans, the future of Alzheimer’s testing could very well center around the lab.Several new and emerging blood tests aim to detect Alzheimer’s biomarkers in a less invasive and more accessible way.
Early diagnosis and intervention can help to improve outcomes for individuals living with Alzheimer’s disease, as well as for their caregivers and families. Currently, the diagnosis of Alzheimer’s disease is made following subjective symptom reporting, cognitive testing, and a series of lab tests that rule out reversible or treatable conditions that can cause cognitive changes. Other testing includes imaging studies such as computerized tomography (CT) scans, magnetic resonance imaging (MRI), and/or positron emission tomography (PET) scans. However, changes in the brain may occur long before clinical symptoms are detected, making early diagnosis of Alzheimer’s disease particularly challenging. Alzheimer’s disease progresses on a spectrum of 3 phases: preclinical, mild cognitive impairment, and then dementia, which is further stratified into mild, moderate, and severe stages. How quickly patients progress through the continuum varies and is influenced by factors including age, environmental factors, genetics, and sex.1A significant step forward in addressing the urgent need for earlier and more accurate detection of Amyloid pathology, a hallmark of Alzheimer’s disease.

BECKMAN COULTER INTRODUCES NEW
Blood-based Neurology Disease Assays (for Research Use Only)
p-Tau217 I GFAP I NfL I NEW APOE ε4 Assays
And a NEW Fully Automated APOE ε4 Testing > Read the study
The APOE (Apolipoprotein E) gene is a critical genetic factor in AD, with its ε4 allele being the strongest known genetic risk factor for AD. APOE is involved in lipid metabolism and plays a crucial role in the clearance of β-amyloid (Aβ), a protein that forms plaques in the brains of AD patients. The presence of the ε4 allele is associated with an increased risk of amyloid accumulation, neuroinflammation, and tau pathology, which collectively contribute to neurodegeneration. Despite its central role in AD, the relationship between APOE and AD remains an active area of research as scientists strive to better understand the mechanisms involved and develop personalized strategies for prevention and treatment.
This new BECKMAN COULTER APOE ε4 RUO blood-based immunoassay offers >99% concordance with PCR genotyping—in only 20 minutes. While not diagnostic, APOE ε4 status can provide insights into risk of developing Alzheimer’s disease.
- Read the study: In this study, scientists determined the sensitivity, imprecision, and concordance with PCR of a new APOE ε4 zygosity RUO assay on the Beckman Coulter DxI 9000 and Access 2 Immunoassay Analyzers.
- White Paper: The Role of APOE in Alzheimer's Disease: Genetic Variants, Mechanisms, and Therapeutic Implications - by Kinal Bhatt, MD, MPH
These neurogenerative RUO assays are all immediately available for use on the DxI 9000 Immunoassay Analyzer
RUO Assays in Development :
ß-AmyIoid 1-42 I BD Tau I
MTBR-Tau I
TDP-43
NEW APOE ε4 Assays Blood-based Neurology Disease Assays
Access APOE ε 4 (RUO) assay (REF D16691) for use with the Access 2 Immunoassay System and the DxI 9000 Immunoassay Analyzer
Information Bulletin
p-Tau217 Blood-based Neurology Disease Assays
Access p-Tau217 (RUO) assay (REF D14230) for use with the DxI 9000 Immunoassay Analyzer
GFAP Assays Blood-based Neurology Disease Assays
Access GFAP (RUO) assay (REF D11672) for use with the Access 2 Immunoassay System and the DxI 9000 Immunoassay Analyzer
Information Bulletin
NfL Assays Blood-based Neurology Disease Assays
Access NfL (RUO) assay (REF D15673) for use with the Access 2 Immunoassay
System and the DxI 9000 Immunoassay Analyzer
Information Bulletin
Neurology Blood Tests Improve Diagnosis, Management of Patients in Alzheimer's and Beyond
Beckman Coulter Life Sciences, T-NeuroDx Collaborate on Early Alzheimer's Diagnostics
The collaboration will combine T-NeuroDx's research in Alzheimer'sdiagnostics with Beckman Coulter's flow cytometry products and is intended to demonstrate the potential of the test to detect an initiating event before dysregulated beta-amyloid and tau, the current standard of care for Alzheimer's biomarker-based diagnostics, are detectable, the company said in a statement.
The neuroimmunology field has long been riddled with complexities which stood in the way of significant advancements, and the introduction of streamlined flow cytometry assays could offer promising new potential for translational research," said our Jean-Marc Busnel, Senior Staff Research Scientist.
WHITE PAPER
Alzheimer’s disease:
Challenges to diagnosis
and the emerging treatment landscape
WHITE PAPER
The Role of APOE in Alzheimer's Disease: Genetic Variants, Mechanisms, and Therapeutic Implications
STUDY
High-throughput, fully automated immunoasday for detecting APOE ε4 zygosity in plasma EDTA:
In this study, scientists determined the sensitivity, imprecision, with PCR of a new APOE ε4 zygosity RUO assay
WHITE PAPER
Blood-Based Biomarkers and the Future of Alzheimer’s Disease Diagnostics
Why blood-based biomarkers for Alzheimer’s disease?
How do blood-based biomarkers for Alzheimer’s disease work?